HanchorBio Presents First-in-Human Data of HCB101 Monotherapy in Relapsed/Refractory Non-Hodgkin Lymphoma at ASH 2025
Early Phase 1 monotherapy data demonstrate cytopenia-sparing safety, broad pharmacologic window, and early clinical activity in relapsed/refractory non-Hodgkin lymphoma (NHL)
HanchorBio Inc. (TPEx: 7827), a global clinical-stage biotechnology company advancing next-generation immunotherapies for oncology and autoimmune diseases, presented new first-in-human data from its ongoing Phase 1 monotherapy study of HCB101, a 3.5th-generation SIRPα-Fc fusion protein, in relapsed/refractory non-Hodgkin lymphoma (R/R NHL) at the 67th Annual Meeting of the American Society of Hematology (ASH), held December 6-9, 2025, in Orlando, Florida.

The ASH Annual Meeting is the world’s premier platform for clinical and translational advances in hematology. This year, over 8,200 abstracts were accepted globally, reaffirming ASH’s position as one of the most competitive and influential medical congresses in hematology and oncology, with historical rejection rates of approximately 28%.
The accepted abstract (#3299) features a focused sub-analysis from HanchorBio’s ongoing multinational, open-label Phase 1 study (NCT05892718) evaluating HCB101 monotherapy across solid and hematologic malignancies, highlighting results from the R/R NHL cohort.
Key Results (data cutoff: October 14, 2025):
- Thirteen patients with R/R NHL received HCB101 (1.28 – 24.0 mg/kg QW). No dose-limiting toxicities (DLTs) were observed, and the maximum tolerated dose (MTD) was not reached.
- All treatment-related adverse events were Grade 1-2, confirming a cytopenia-sparing safety profile.
- CD47 receptor occupancy (RO) reached ≥ 75-85% at 12 mg/kg and ≥ 90% at 8 mg/kg, demonstrating a broad pharmacologic window.
- A confirmed partial response (PR) was observed in a patient with marginal zone B-cell lymphoma at 8.00 mg/kg, with -43.3% tumor reduction at Week 8, deepening to -89.5% by Week 16 at the same dose.
The results were presented by Alvin Luk, PhD, MBA, CCRA, President & Chief Medical Officer and Chief Executive Officer (U.S.) of HanchorBio during the ASH 2025 poster session. Dr. Luk recently joined HanchorBio to lead the company’s global development of late-stage products and U.S. operations, advancing its next-generation immuno-oncology pipeline.
“Despite ASH’s highly competitive selection process, the inclusion of HCB101 monotherapy data reflects the strong translational foundation and clinical potential of our SIRPα-CD47 backbone,” said Scott Liu, Ph.D., Founder, Chairman, and Chief Executive Officer of HanchorBio. “We’re encouraged by the favorable safety and early signs of clinical activity seen in the heavily pretreated patient population. These findings validate the translational strength of our FBDB™ platform, and we look forward to engaging with the global hematology community as we expand HCB101 into hematologic malignancies and macrophage/T-cell combination strategies.”
“Presenting these data at ASH marks an important step for HanchorBio and our team,” added Dr. Alvin Luk. “Moving from molecular design to early clinical validation highlights the potential of selective SIRPα-CD47 blockade to achieve both safety and effective immune activation in patients with limited treatment options.”
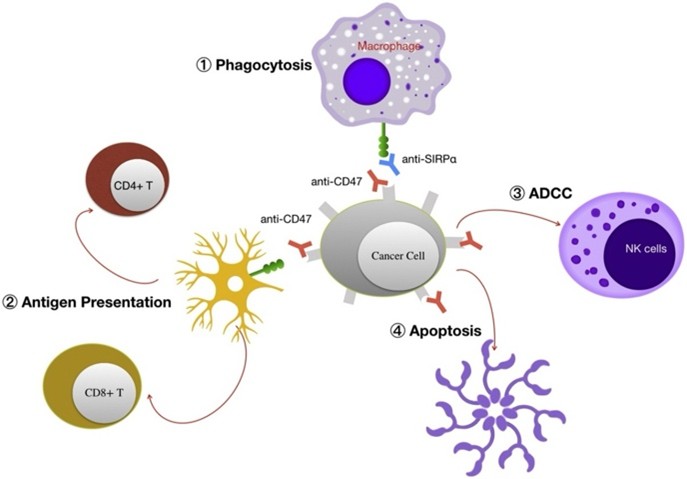
About HCB101: A Differentiated CD47-SIRPα Blockade
HCB101 is a 3.5th-generation, affinity-optimized SIRPα-Fc fusion protein with an intact IgG4 Fc backbone, developed using HanchorBio’s proprietary FBDB™ platform. It is engineered for selective CD47 targeting with low red blood cell (RBC) binding, thereby avoiding the anemia and thrombocytopenia commonly associated with earlier anti-CD47 monoclonal antibodies, while preserving strong antibody-dependent cellular phagocytosis (ADCP) and innate-to-adaptive immune bridging. Key differentiators of HCB101:
- Enhanced safety: Cytopenia-sparing profile, with no DLTs observed up to 30 mg/kg and receptor occupancy >90% at ≥28 mg/kg, supporting a broad therapeutic window.
- Robust immune activation: Engineered to enhance ADCP and bridge innate-to-adaptive immunity, with evidence of durable immune-mediated tumor control in monotherapy.
- Broad tumor applicability: Demonstrated activity across >80 PDX and CDX preclinical models, with early clinical signals in gastric cancer, TNBC, HNSCC, non-Hodgkin lymphoma, and ovarian cancer.
- Clinical translation: Shows durable disease control as monotherapy and a 100% confirmed partial response rate (6/6) in 2L gastric cancer when combined with ramucirumab and paclitaxel, with additional confirmed responses in 1L TNBC and 2L HNSCC, substantially exceeding historical benchmarks.
About HanchorBio
Based in Taipei, Shanghai, and the San Francisco Bay Area, HanchorBio (TPEx: 7827) is a global biotechnology company specializing in immuno-oncology. It is led by an experienced team of pharmaceutical industry veterans with a proven track record in biologics discovery and international development, aiming to rewrite the landscape of cancer therapies. Committed to reactivating the immune system to fight diseases, the proprietary Fc-based designer biologics (FBDB™) platform enables the development of unique biologics with diverse multi-targeting modalities, unleashing both innate and adaptive immunity to overcome the current challenges of anti-PD1/L1 therapies. The FBDB™ platform has successfully delivered proof-of-concept data in several in vivo tumor animal models. By advancing breakthroughs in multi-functional, innovative molecular configurations in R&D and improving CMC manufacturing processes, HanchorBio develops transformative medicines to address unmet medical needs.
漢康生技於 2025 年美國血液學會(ASH)年會發表 HCB101 單藥治療人體試驗結果
一期早期單藥臨床數據展現優異安全性、廣泛藥理窗口與在復發/難治性非何杰金氏淋巴瘤患者中的早期療效訊號
全球臨床階段生技公司 漢康生技(HanchorBio Inc., TPEx: 7827) 於 2025 年美國血液學會(ASH)第 67 屆年會,發表旗下 3.5 代 SIRPα-Fc 融合蛋白 HCB101 在復發/難治性非何杰金氏淋巴瘤(R/R NHL)的一期臨床單藥治療人體試驗數據。
ASH 年會為全球血液學領域最重要的臨床與轉譯研究平台,今年共收到超過 8,200 件投稿,再次展現其高度競爭性與國際影響力,歷年論文拒絕率約為 28%。
此次入選的摘要(#3299)來自漢康正在進行的多國、多中心、開放式一期臨床試驗(NCT05892718),針對 HCB101 單藥在多項實體與血液腫瘤中的研究,並特別呈現復發/難治性 NHL 族群的最新結果。
主要結果(數據截止:2025 年 10 月 14 日)
- 共有 13 位 R/R NHL 患者接受 HCB101(劑量範圍 1.28–24.0 mg/kg,每週給藥一次)。
未觀察到劑量限制性毒性(DLTs),且尚未達到最大耐受劑量(MTD)。 - 所有治療相關不良事件均為 1–2 級,證實 不產生血球毒性的安全特性(cytopenia-sparing)。
- CD47 受體佔有率(RO)於5.12 mg/kg 即達 ≥75-85%,於 8 mg/kg 達 ≥90%,展示廣泛的藥理窗口。
- 在一名邊緣區 B 細胞淋巴瘤患者中觀察到 確定的部分緩解(PR):
- 第 8 週腫瘤縮小 -43.3%
- 第 16 週加深至 -89.5%(劑量 8.0 mg/kg)
研究結果由漢康生技醫療長的陸英明 博士於 ASH 2025 海報展示中發表。陸博士近期加入漢康,負責推動公司後期產品的全球開發及美國業務,並加速推進下一代免疫腫瘤治療產品線。
ASH 的選稿非常競爭,HCB101 單藥數據成功入選,突顯 SIRPα-CD47技術強大的轉譯基礎與臨床潛力。這些結果再次驗證了漢康 FBDB™ 技術平台的實力。漢康將與全球血液學界有更深入的交流,並持續拓展 HCB101 在血液腫瘤以及巨噬細胞/ T 細胞組合療法中的發展。
這是從分子設計走向人體臨床驗證的過程,證明了選擇性 SIRPα-CD47 阻斷有機會在兼具安全性的同時,達到有效的免疫活化,為治療選擇有限的患者帶來新的希望。
關於 HCB101:差異化的 CD47-SIRPα 阻斷機制藥物
HCB101 是一款 3.5 代、經親和力優化的 SIRPα-Fc 融合蛋白,保留完整 IgG4 Fc 結構,由漢康生技自有 FBDB™ 平台開發。
其設計目標為 選擇性 CD47 靶向、低紅血球(RBC)結合,避免過往 CD47 單抗常見的貧血與血小板下降問題,同時維持強勁的抗體依賴性細胞吞噬(ADCP)與先天/適應性免疫橋接能力。
HCB101 的關鍵差異化特色:
- 增強安全性:
展現不造成血球毒性的特性,至 30 mg/kg 未見 DLT,且在 ≥1.28 mg/kg 即達 >90% 受體佔有率,支持極具吸引力的治療窗口。 - 強大的免疫活化能力:
能加強 ADCP 並促進先天至適應性免疫的橋接,在單藥治療中展示可持續的免疫介導腫瘤控制證據。 - 廣泛的腫瘤適應性:
在超過 80 個 PDX/CDX 前臨床模型中展現活性,並於胃癌、TNBC、HNSCC、NHL、卵巢癌等適應症中觀察到早期臨床療效訊號。
臨床轉譯成果突出:
單藥可達持續疾病控制;與雷莫蘆單抗+紫杉醇聯用於二線胃癌可達 100% 確認部分緩解(6/6);並在 1L TNBC、2L HNSCC 中觀察到額外確定反應,均大幅超越歷史基準。



