HanchorBio Reports High Objective Response Rate with HCB101 Combination in Second-Line Gastric Cancer Following ASCO-GI 2026 Poster Presentation
Mid-dose cohorts demonstrate ~80% ORR when HCB101 is layered on the standard ramucirumab and paclitaxel in second-line gastric cancer.
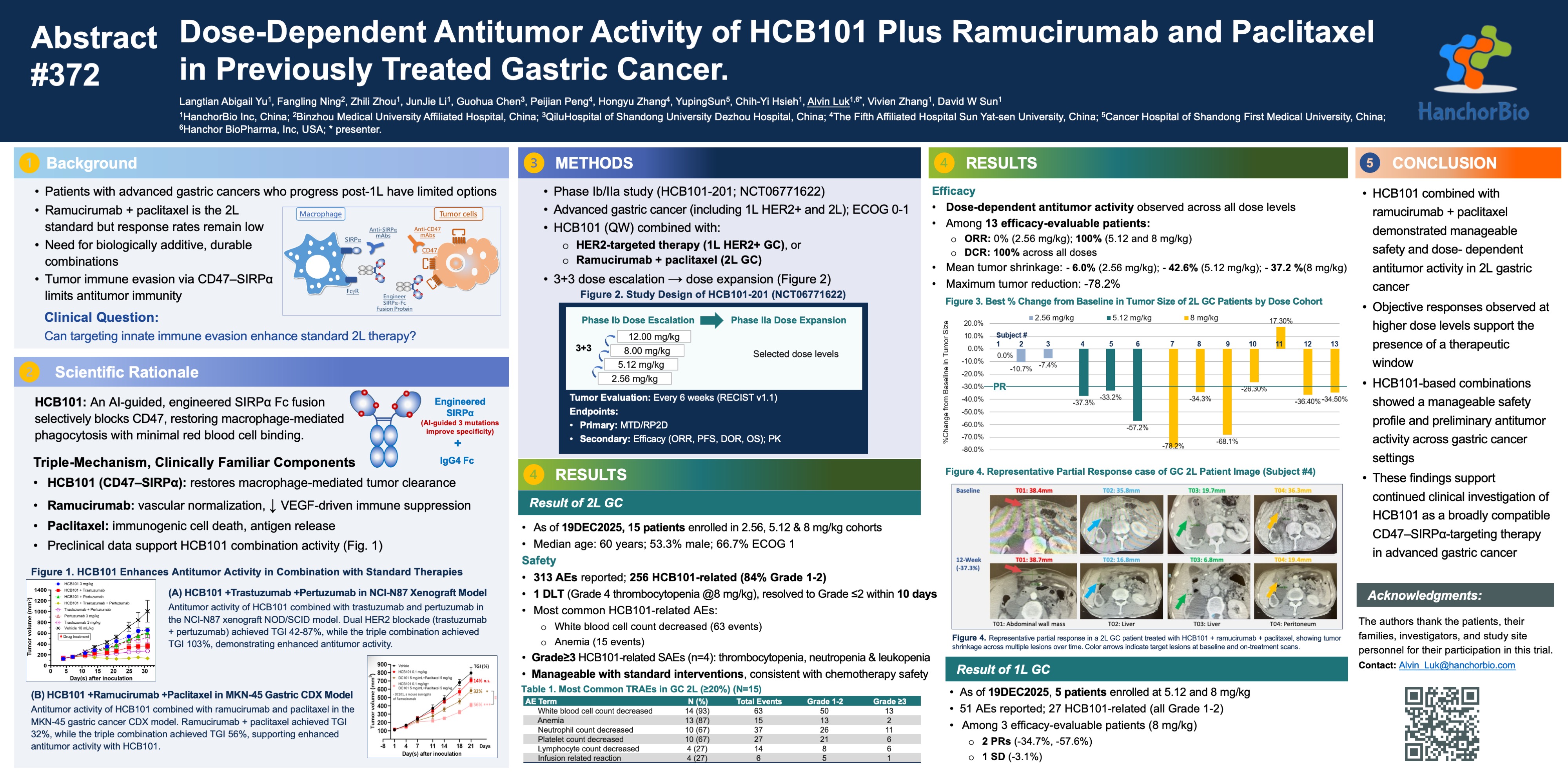
HanchorBio, Inc. (TPEx: 7827), a global clinical-stage biotechnology company advancing next-generation immunotherapies for oncology and autoimmune diseases, today reported updated clinical data from its ongoing Phase Ib/IIa study evaluating HCB101 in combination with ramucirumab and paclitaxel in patients with previously treated gastric cancer. The data were presented on January 8, 2026, at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium (ASCO-GI) 2026 in San Francisco. The abstract was accepted as a Trial in Progress poster, representing approximately 9.7% of all accepted abstracts at ASCO-GI 2026. The abstract was accepted as a Trial In-Progress poster, representing approximately 9.7% of all accepted abstracts at ASCO-GI 2026.
Key Clinical Findings – Second-Line Gastric Cancer
As of the most recent data cutoff, 13 patients with second-line gastric adenocarcinoma were efficacy-evaluable across dose levels:
Overall (all doses):
- Overall Objective Response Rate (ORR) all dose levels (a low & two mid-doses): ~58.3% (7/12)
- Disease Control Rate (DCR): 100%
- Mid-dose cohorts (5.12 and 8.0 mg/kg) ORR: 80% (8/10)
- Maximum tumor burden reduction (SoD): -78%
- Multiple responses sustained beyond 18 weeks (historical benchmarks of ramucirumab plus paclitaxel of ~4.1 to 4.4 months), with ongoing treatment in several patients.
While cross-trial comparisons should be interpreted cautiously, these response rates compare favorably with historical outcomes for standard second-line therapy, as established by the global RAINBOW (NCT01170663) and RAINBOW-Asia (NCT01939899) trials.
“The CD47–SIRPα axis has long been recognized as a powerful innate immune checkpoint, but translating that biology into reproducible clinical benefit has been challenging,” said Scott Liu, PhD, Founder, Chairman, and CEO of HanchorBio. “These ASCO-GI data provide a concrete clinical example that macrophage checkpoint modulation, when engineered correctly, can be integrated into validated, commercially established oncology treatment regimens and deliver meaningful antitumor activity. This reinforces our conviction that HCB101 can function as a combination-ready innate immune backbone with broad potential across solid tumors.”
Mechanistic Rationale
HCB101 is an engineered SIRPα–Fc fusion protein that selectively blocks the CD47–SIRPα innate immune checkpoint on tumor cells, thereby restoring macrophage-mediated tumor clearance while minimizing red blood cell binding. In gastric cancer, the tumor microenvironment is characterized by macrophage polarization, VEGF-driven immune suppression, and CD47-mediated innate immune evasion.
As illustrated in the accompanying schematic (Mou P. et al., Frontier Immunology 2023) of the gastric cancer immune microenvironment, blockade of CD47–SIRPα enables re-engagement of macrophage-mediated phagocytosis and downstream adaptive immune activation. In combination, ramucirumab, a cornerstone of second-line gastric cancer treatment, promotes vascular normalization and reduces VEGF-driven immunosuppression, while paclitaxel induces immunogenic tumor cell death and antigen release. Together, this combination enables innate immune activation into a validated standard-of-care therapy, providing a biologically coherent and clinically practical framework for the antitumor activity observed in 2L gastric cancer.
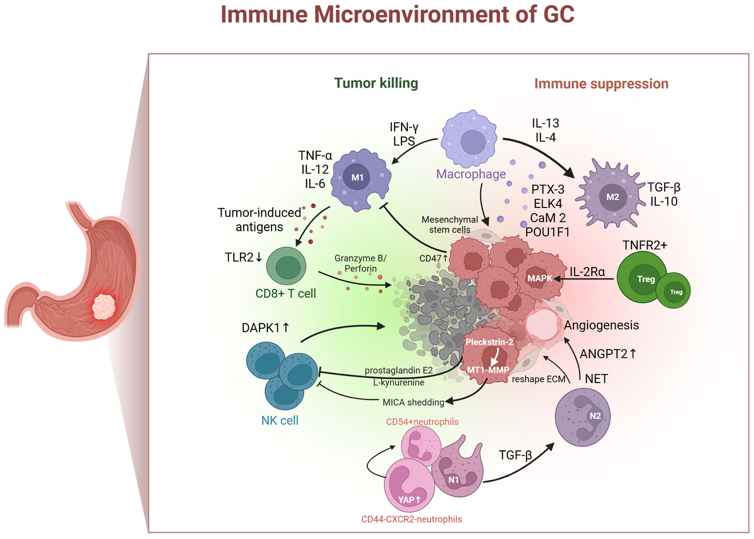
Figure: Immune microenvironment of gastric cancer highlighting macrophage polarization, VEGF-driven immune suppression, and CD47–SIRPα-mediated innate immune evasion.
Alvin Luk, PhD, MBA, CCRA, President & Chief Medical Officer (Group) and Chief Executive Officer (U.S.A.) of HanchorBio, added, “What is particularly compelling about these updated data is not only the response rate, but the consistency of tumor shrinkage and disease control observed at clinically relevant dose levels. In a second-line setting where historical ORRs with ramucirumab plus paclitaxel are typically in the mid-20% range, observing investigator-assessed response rates approaching 80% while maintaining compatibility with standard ramucirumab-paclitaxel administration is highly encouraging. These findings support continued dose optimization and further clinical development of this combination.”
About HCB101
HCB101 is a rationally engineered SIRPα–IgG4 Fc fusion protein developed on HanchorBio’s FBDB™ platform to selectively block the CD47–SIRPα innate immune checkpoint while minimizing hematologic toxicity. Importantly, HCB101 was designed to be compatible with combinations, enabling innate immune engagement without disrupting standard dosing, safety expectations, or clinical workflows. Across clinical and translational studies, HCB101 has demonstrated consistent target engagement and early antitumor activity as monotherapy and in combination, including in tumor settings historically considered challenging for CD47-directed therapies. Together, these attributes position HCB101 as a differentiated macrophage checkpoint backbone with broad potential for combination strategies across validated treatment frameworks in solid tumors and hematologic malignancies.
About HanchorBio
Based in Taipei, Shanghai, and the San Francisco Bay Area, HanchorBio (TPEx: 7827) is a global biotechnology company specializing in immuno-oncology. It is led by an experienced team of pharmaceutical industry veterans with a proven track record in biologics discovery and international development, aiming to reshape the landscape of cancer therapies. Committed to reactivating the immune system to fight disease, the proprietary Fc-based designer biologics (FBDB™) platform enables the development of unique biologics with diverse multi-targeting modalities, unleashing both innate and adaptive immunity to overcome the current challenges of anti-PD1/L1 therapies. The FBDB™ platform has successfully delivered proof-of-concept data in several in vivo tumor animal models. By advancing breakthroughs in multi-functional, innovative molecular configurations in R&D and improving CMC manufacturing processes, HanchorBio develops transformative medicines to address unmet medical needs.



