HanchorBio Reports High Objective Response Rate with HCB101 Combination in Second-Line Gastric Cancer Following ASCO-GI 2026 Poster Presentation
Mid-dose cohorts demonstrate ~80% ORR when HCB101 is layered on the standard ramucirumab and paclitaxel in second-line gastric cancer.
HanchorBio, Inc. (TPEx: 7827), a global clinical-stage biotechnology company advancing next-generation immunotherapies for oncology and autoimmune diseases, today reported updated clinical data from its ongoing Phase Ib/IIa study evaluating HCB101 in combination with ramucirumab and paclitaxel in patients with previously treated gastric cancer. The data were presented on January 8, 2026, at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium (ASCO-GI) 2026 in San Francisco. The abstract was accepted as a Trial in Progress poster, representing approximately 9.7% of all accepted abstracts at ASCO-GI 2026. The abstract was accepted as a Trial In-Progress poster, representing approximately 9.7% of all accepted abstracts at ASCO-GI 2026.
Key Clinical Findings – Second-Line Gastric Cancer
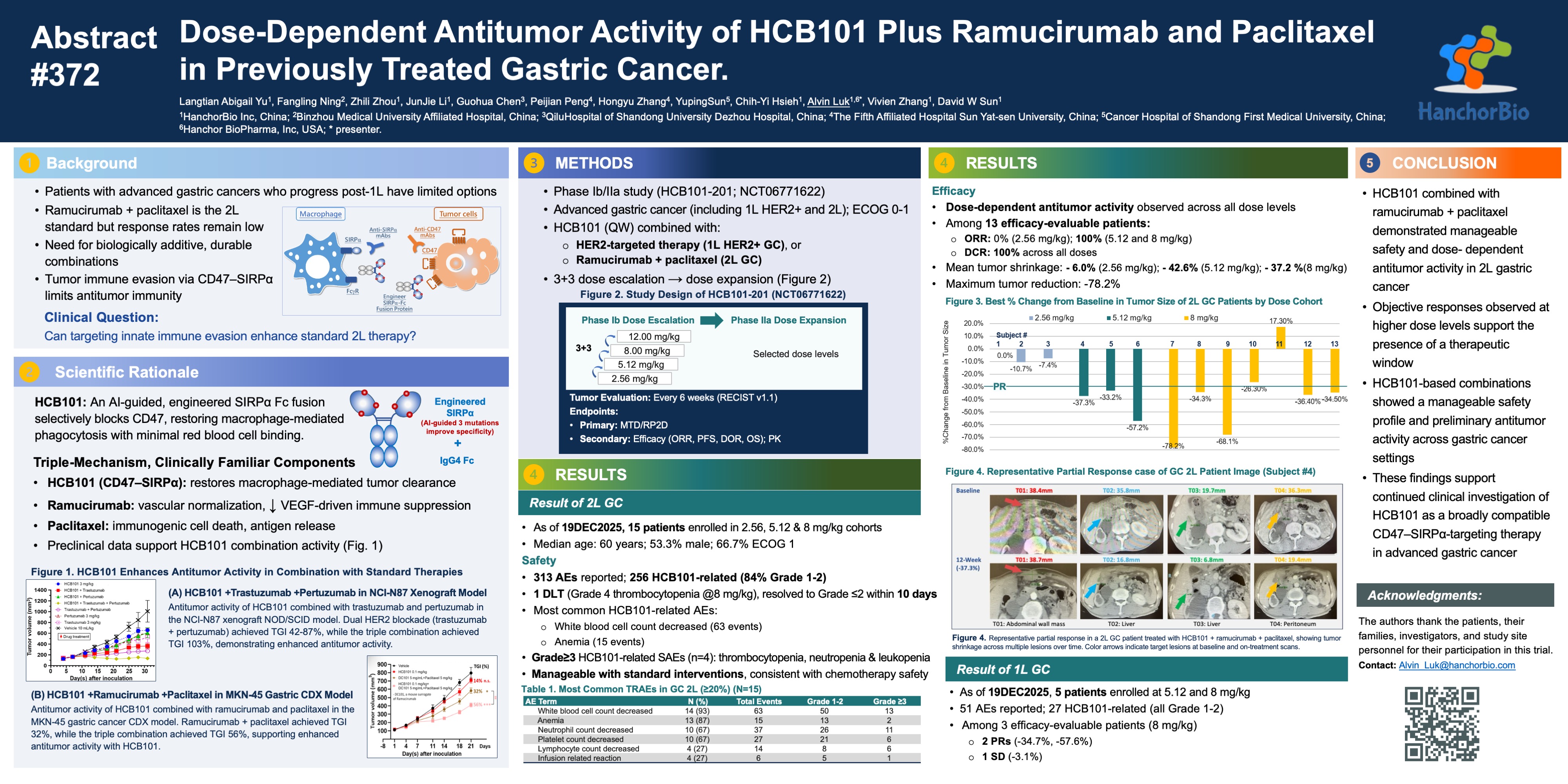
As of the most recent data cutoff, 13 patients with second-line gastric adenocarcinoma were efficacy-evaluable across dose levels:
Overall (all doses):
- Overall Objective Response Rate (ORR) all dose levels (a low & two mid-doses): ~58.3% (7/12)
- Disease Control Rate (DCR): 100%
- Mid-dose cohorts (5.12 and 8.0 mg/kg) ORR: 80% (8/10)
- Maximum tumor burden reduction (SoD): -78%
- Multiple responses sustained beyond 18 weeks (historical benchmarks of ramucirumab plus paclitaxel of ~4.1 to 4.4 months), with ongoing treatment in several patients.
While cross-trial comparisons should be interpreted cautiously, these response rates compare favorably with historical outcomes for standard second-line therapy, as established by the global RAINBOW (NCT01170663) and RAINBOW-Asia (NCT01939899) trials.
“The CD47–SIRPα axis has long been recognized as a powerful innate immune checkpoint, but translating that biology into reproducible clinical benefit has been challenging,” said Scott Liu, PhD, Founder, Chairman, and CEO of HanchorBio. “These ASCO-GI data provide a concrete clinical example that macrophage checkpoint modulation, when engineered correctly, can be integrated into validated, commercially established oncology treatment regimens and deliver meaningful antitumor activity. This reinforces our conviction that HCB101 can function as a combination-ready innate immune backbone with broad potential across solid tumors.”
Mechanistic Rationale
HCB101 is an engineered SIRPα–Fc fusion protein that selectively blocks the CD47–SIRPα innate immune checkpoint on tumor cells, thereby restoring macrophage-mediated tumor clearance while minimizing red blood cell binding. In gastric cancer, the tumor microenvironment is characterized by macrophage polarization, VEGF-driven immune suppression, and CD47-mediated innate immune evasion.
As illustrated in the accompanying schematic (Mou P. et al., Frontier Immunology 2023) of the gastric cancer immune microenvironment, blockade of CD47–SIRPα enables re-engagement of macrophage-mediated phagocytosis and downstream adaptive immune activation. In combination, ramucirumab, a cornerstone of second-line gastric cancer treatment, promotes vascular normalization and reduces VEGF-driven immunosuppression, while paclitaxel induces immunogenic tumor cell death and antigen release. Together, this combination enables innate immune activation into a validated standard-of-care therapy, providing a biologically coherent and clinically practical framework for the antitumor activity observed in 2L gastric cancer.
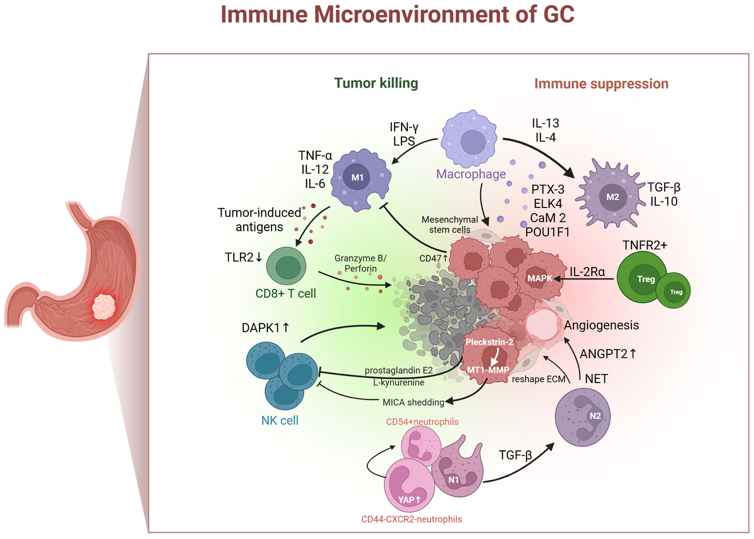
Figure: Immune microenvironment of gastric cancer highlighting macrophage polarization, VEGF-driven immune suppression, and CD47–SIRPα-mediated innate immune evasion.
Alvin Luk, PhD, MBA, CCRA, President & Chief Medical Officer (Group) and Chief Executive Officer (U.S.A.) of HanchorBio, added, “What is particularly compelling about these updated data is not only the response rate, but the consistency of tumor shrinkage and disease control observed at clinically relevant dose levels. In a second-line setting where historical ORRs with ramucirumab plus paclitaxel are typically in the mid-20% range, observing investigator-assessed response rates approaching 80% while maintaining compatibility with standard ramucirumab-paclitaxel administration is highly encouraging. These findings support continued dose optimization and further clinical development of this combination.”
About HCB101
HCB101 is a rationally engineered SIRPα–IgG4 Fc fusion protein developed on HanchorBio’s FBDB™ platform to selectively block the CD47–SIRPα innate immune checkpoint while minimizing hematologic toxicity. Importantly, HCB101 was designed to be compatible with combinations, enabling innate immune engagement without disrupting standard dosing, safety expectations, or clinical workflows. Across clinical and translational studies, HCB101 has demonstrated consistent target engagement and early antitumor activity as monotherapy and in combination, including in tumor settings historically considered challenging for CD47-directed therapies. Together, these attributes position HCB101 as a differentiated macrophage checkpoint backbone with broad potential for combination strategies across validated treatment frameworks in solid tumors and hematologic malignancies.
About HanchorBio
Based in Taipei, Shanghai, and the San Francisco Bay Area, HanchorBio (TPEx: 7827) is a global biotechnology company specializing in immuno-oncology. It is led by an experienced team of pharmaceutical industry veterans with a proven track record in biologics discovery and international development, aiming to reshape the landscape of cancer therapies. Committed to reactivating the immune system to fight disease, the proprietary Fc-based designer biologics (FBDB™) platform enables the development of unique biologics with diverse multi-targeting modalities, unleashing both innate and adaptive immunity to overcome the current challenges of anti-PD1/L1 therapies. The FBDB™ platform has successfully delivered proof-of-concept data in several in vivo tumor animal models. By advancing breakthroughs in multi-functional, innovative molecular configurations in R&D and improving CMC manufacturing processes, HanchorBio develops transformative medicines to address unmet medical needs.
漢康生技於2026 ASCO GI消化道腫瘤大會發表 HCB101 聯合標準治療在二線胃癌之臨床數據
中劑量HCB101聯合標準治療 (ramucirumab與paclitaxel) 於二線胃癌中的 ORR 約達 80%
漢康生技(股票代碼:7827),一家致力於開發次世代腫瘤與自體免疫疾病免疫療法的全球臨床階段生技公司,今日公布其正在進行中的 HCB101 聯合標準治療 (SoC) 之 Phase 1b/2a 臨床試驗最新數據,評估 HCB101 聯合 ramucirumab 與 paclitaxel 用於既往治療後胃癌患者之療效表現。
相關數據於 2026 年 1 月 8 日在美國舊金山舉行的美國臨床腫瘤學會消化道腫瘤大會(ASCO-GI 2026)發表。該研究摘要以「進行中試驗(Trial in Progress)」壁報形式獲 ASCO-GI 2026 接受,屬於本屆大會中約 9.7% 的入選摘要之一。
臨床重點數據-二線胃癌
截至最近一次資料截點,共有 13 位二線胃腺癌患者於不同劑量組中具備療效評估資格:
整體(所有劑量):
– 整體客觀反應率(ORR):約 58.3%(7/12)
– 疾病控制率(DCR):100%
中劑量組(5.12 與 8.0 mg/kg):
– ORR:80%(8/10)
– 最大腫瘤負荷縮小幅度(SoD):-78%
– 多位患者之反應持續超過 18 週,部分患者目前仍持續接受治療(相較於歷史上 ramucirumab + paclitaxel 之中位無惡化存活期約 4.1–4.4 個月)
整體而言,儘管跨試驗比較需審慎解讀,上述療效結果與全球 RAINBOW(NCT01170663)及 RAINBOW-Asia(NCT01939899)試驗所建立之二線標準治療歷史數據相比,顯示出具競爭力的臨床表現。
漢康生技創辦人、董事長暨執行長劉世高博士表示:「CD47–SIRPα 通路長期以來被視為關鍵的先天免疫檢查點,但要此生物學機制穩定轉化為臨床可重現的療效,一直具有挑戰性。本次 ASCO-GI 所呈現的數據提供了具體的臨床例證,顯示在經過合理工程設計後,巨噬細胞檢查點調控可成功整合至既有、已商業化的腫瘤治療架構中,產生具意義的抗腫瘤活性。這也進一步支持HCB101作為可用於聯合治療的先天免疫骨幹藥物的信心,在多種實體瘤中具備發展潛力。」
作用機轉基礎
HCB101 為一款工程化 SIRPα–IgG4 Fc 融合蛋白,可選擇性阻斷 CD47–SIRPα先天免疫檢查點,恢復巨噬細胞對腫瘤細胞的吞噬作用,同時將紅血球結合風險降至最低。在胃癌中,腫瘤微環境常呈現巨噬細胞極化、VEGF 驅動之免疫抑制,以及 CD47 介導的先天免疫逃脫。
如文獻所示 (Mou P, et al., Frontier Immunology 2023),阻斷 CD47–SIRPα 通路可重新啟動巨噬細胞吞噬功能,並促進後續的適應性免疫反應。
在本研究之聯合治療策略中:
- Ramucirumab 作為二線胃癌治療的核心藥物,有助於血管恢復正常化並降低 VEGF相關免疫抑制
- Paclitaxel 可誘發具免疫原性的腫瘤細胞死亡,促進抗原釋放
三者聯用,使先天免疫活化得以自然整合至既有的標準治療體系,形成兼具生物學合理性且臨床可執行的抗腫瘤策略。

胃癌腫瘤微環境,說明巨噬細胞極化、VEGF 介導的免疫抑制,以及 CD47–SIRPα 介導的先天免疫逃脫。
漢康生技集團總裁暨醫療長、及美國子公司執行長 陸英明 博士 補充表示:
「本次更新數據特別令人關注的部分,不僅是客觀反應率本身,更在於具臨床意義的劑量水準下,所觀察到的腫瘤縮小表現與疾病控制的一致性。在二線治療情境中,ramucirumab 聯合 paclitaxel 的歷史客觀反應率通常僅落在約20% 出頭,然而在本研究中,在維持與既有 ramucirumab–paclitaxel給藥方式相容、不影響標準治療流程的前提下,觀察到接近 80%的研究者評估反應率,相當令人鼓舞。這些結果支持持續進行劑量優化,並推進此一聯合治療方案的後續臨床開發。
關於 HCB101
HCB101 是一款基於漢康生技自主開發 FBDB™ 平台 理性設計的 SIRPα–IgG4 Fc 融合蛋白,旨在選擇性阻斷 CD47–SIRPα 先天免疫檢查點的同時,最大程度降低將血液學毒性。與早期抗 CD47 療法不同,HCB101 的設計能在保留巨噬細胞抗腫瘤活性的同時,顯著減少對紅血球與血小板的非特異性結合,從而突破了該靶點過往臨床開發中的主要限制。
此外,HCB101 在設計之初即以聯合治療為核心目標。其安全性、受體佔據率 (RO) 及藥代動力學特性,支持其在不干擾標準劑量、安全性預期及臨床流程的前提下,與成熟腫瘤治療方案協應用。迄今為止,HCB101 已在單藥及聯合治療的臨床與轉譯研究中,展現出一致的靶點結合能力和早期抗腫瘤活性,包括在過去被認為對 CD47 靶向療法具挑戰性的腫瘤類型中。這些特性使 HCB101 成為一款具差異化潛力的巨噬細胞檢查點骨幹療法,可廣泛應用於實證成熟的實體瘤與血液腫瘤治療框架中。
關於漢康生技
漢康生技總部設於台北,並於上海與美國舊金山灣區設有據點,是一家專注於免疫腫瘤學的全球生技公司(TPEx:7827)。公司由具備豐富生物製劑研發與國際臨床開發經驗的專業團隊領導,致力於重塑癌症治療版圖。
漢康生技以重新活化免疫系統對抗疾病為使命,其專屬的FBDB™ (Fc-based Designer Biologics) 平台 可開發具多重靶點與多功能設計的創新生物藥,協同啟動先天與後天免疫反應,以突破現行抗 PD-1/PD-L1 治療的限制。FBDB™ 平台已在多項體內腫瘤動物模型中成功建立概念驗證 (PoC)。透過在創新分子設計與 CMC 製程上的持續突破,漢康生技致力於開發具轉化意義的創新藥物,以滿足未被滿足的重大醫療需求。



